Imagine looking at Earth from space and being able to listen in on what individuals are saying to each other. That’s about how challenging it is to understand how the brain works.
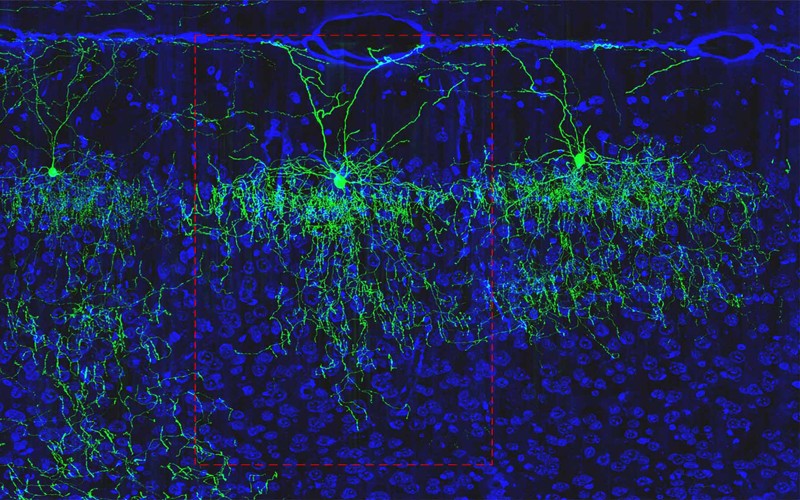
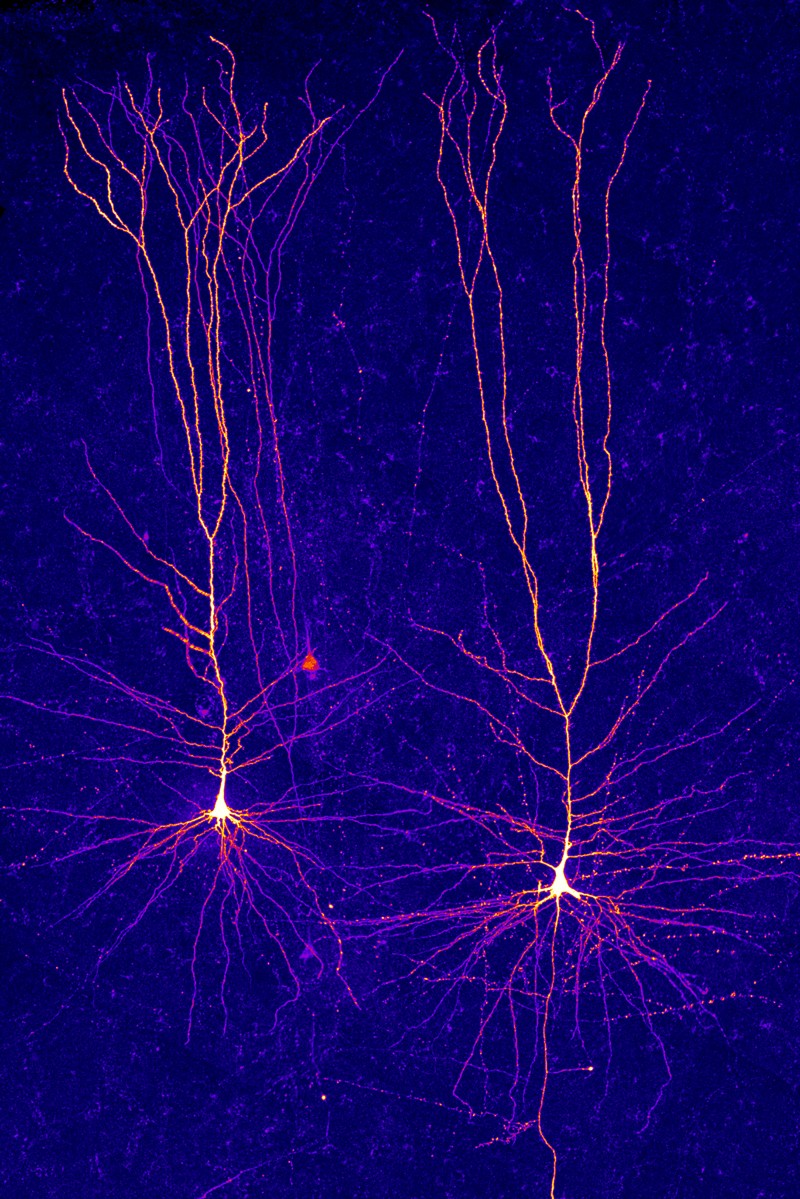
From the organ’s wrinkled surface, zoom in a million-fold and you’ll see a kaleidoscope of cells of different shapes and sizes, which branch off and reach out to each other. Zoom in a further 100,000 times and you’ll see the cells’ inner workings — the tiny structures in each one, the points of contact between them and the long-distance connections between brain areas.
Scientists have made maps such as these for the worm1 and fly2 brains, and for tiny parts of the mouse3 and human4 brains. But those charts are just the start. To truly understand how the brain works, neuroscientists also need to know how each of the roughly 1,000 types of cell thought to exist in the brain speak to each other in their different electrical dialects. With that kind of complete, finely contoured map, they could really begin to explain the networks that drive how we think and behave.
Such maps are emerging, including in a series of papers published this week that catalogue the cell types in the brain. Results are streaming in from government efforts to understand and stem the increasing burden of brain disorders in their ageing populations. These projects, launched over the past decade, aim to systematically chart the brain’s connections and catalogue its cell types and their physiological properties.
It’s an onerous undertaking. “But knowing all the brain cell types, how they connect with each other and how they interact, will open up an entirely new set of therapies that we can’t even imagine today,” says Josh Gordon, director of the US National Institute of Mental Health (NIMH) in Bethesda, Maryland.
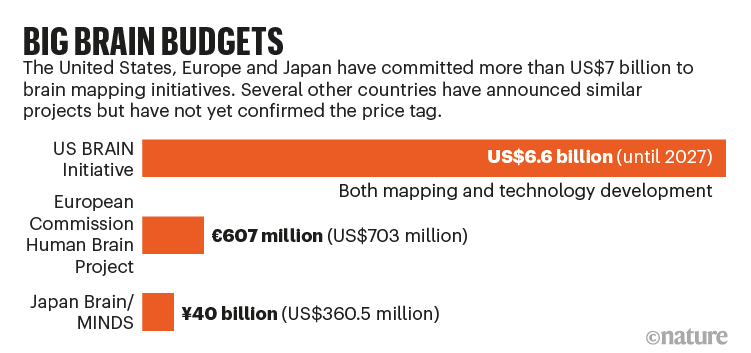
The largest projects started in 2013, when the US government and the European Commission launched ‘moonshot’ efforts to provide services to researchers that will help to crack the mammalian brain’s code. They each poured vast resources into large-scale systematic programmes with different goals. The US effort — which is estimated to cost US$6.6 billion up until 2027 — has focused on developing and applying new mapping technologies in its BRAIN (Brain Research through Advancing Innovative Neurotechnologies) Initiative (see ‘Big brain budgets’). The European Commission and its partner organizations have spent €607 million ($703 million) on the Human Brain Project (HBP), which is aimed mainly at creating simulations of the brain’s circuitry and using those models as a platform for experiments.
Inspired by these efforts, which initially focused on mice, in 2014 Japan launched its Brain/MINDS (Brain Mapping by Integrated Neurotechnologies for Disease Studies) project, a large part of which involves mapping neural networks in the marmoset brain. Since then, other countries, including Canada, Australia, South Korea and China, have launched or pledged to launch generous brain-science programmes with more-distributed aims.
These works-in-progress are already generating colossal — and diverse — data sets, all of which will be open to the community. In December 2020, for example, the HBP launched its EBRAINS platform to provide access to data sets on various scales, the digital tools to analyse them and the resources to conduct experiments (https://ebrains.eu).
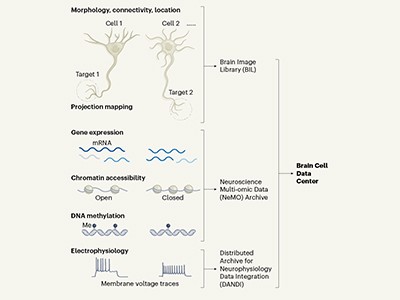
One of the largest and best-funded efforts, bankrolled by the BRAIN Initiative, is a giant catalogue of cell types being created by the BRAIN Initiative Cell Census Network (BICCN), a consortium of 26 teams in US research institutions. The catalogue describes how many different brain cell types there are, in what proportions they exist and how they are spatially arranged.
“Understanding the brain requires knowledge of its basic elements and how they are organized,” says BICCN member Josh Huang, a neurobiologist at Duke University in Durham, North Carolina. “It’s our starting point for figuring out how a neural circuit is built and functions — and ultimately to understanding the complex behaviours those circuits drive.”
The BICCN is publishing a tranche of 17 papers in Nature on 7 October; several other papers have already been published across the Nature Portfolio. The consortium has mapped the cell types in around 1% of the mouse brain, and has some preliminary data on primate brains, including humans. It plans to complete the whole mouse brain by 2023. The maps already hint at some small differences between species that could help to explain our susceptibility to some human-specific conditions such as Alzheimer’s disease.
Neuroscientists are particularly excited that the BICCN is building tools that target particular cell types and circuits relevant to disease, which will help to test hypotheses about brain function and to develop therapies.
The cell catalogue is a much-needed touchstone, says neuroscientist Christof Koch, president of the Allen Institute for Brain Science in Seattle, Washington. “Nothing in chemistry makes sense without the periodic table, and nothing is going to make sense in understanding the brain without understanding the existence and function of cell types.”
Type hunter
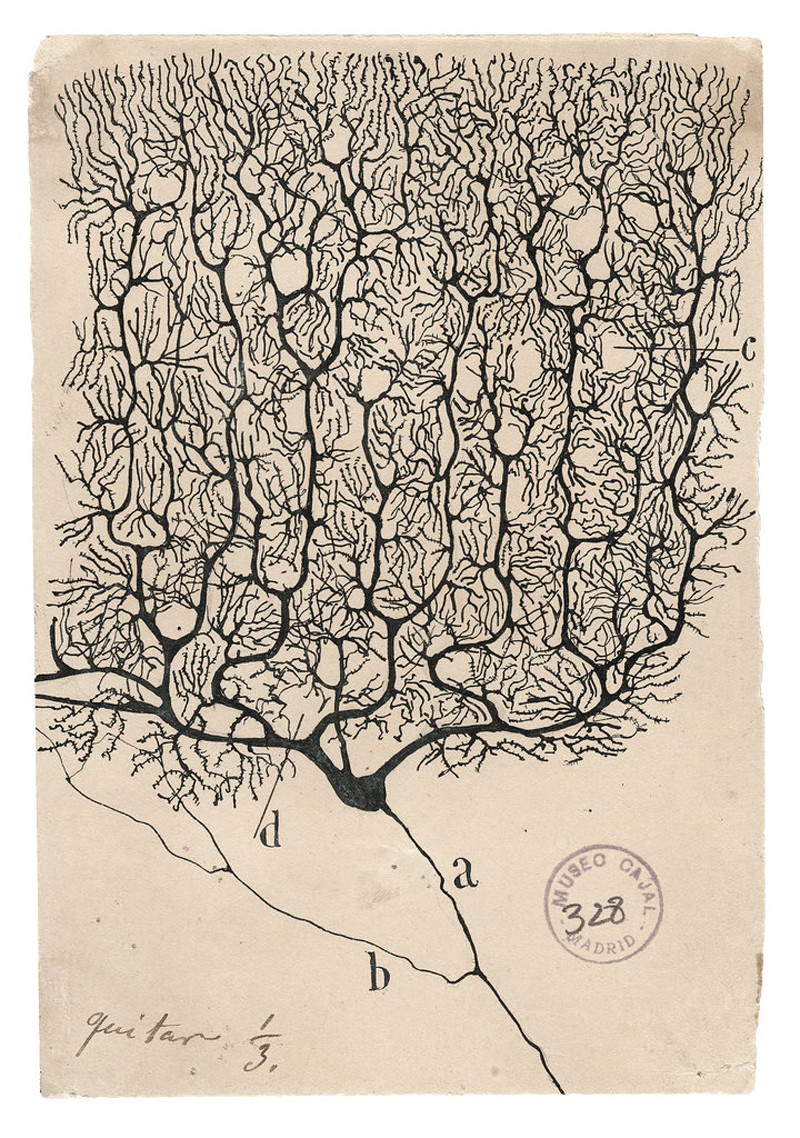
More than a century ago, the Spanish neuroscientist Santiago Ramón y Cajal was the first to show just how many different cell types there were in the mammalian brain. He stained neurons so that they could be seen under a microscope, and then made precise and beautiful drawings of their shapes. Among the few dozen types he found, some had extensions — or axons — that reached out of blobby cell bodies like spiders’ legs over long distances. Some had short axons; others looked more like stars. He deduced that, because the axons of each cell were very close to the cell bodies of others, they were probably transmitting information. He shared the 1906 Nobel Prize in Physiology or Medicine for his discoveries.
Most studies of cell types have since focused on the brain’s cortex, which controls many of an animal’s more sophisticated behaviours. In the past three decades, neuroscientists have worked out that there are three main classes of cell in the cortex, for which the lineages can be traced back to different stages of development. These include two classes of neuron — inhibitory and excitatory. Both transmit electrical pulses, but the first suppresses activity in partner neurons and the other incites it. The third class comprises a huge number of non-neuronal cells that support and protect the neurons.
Over the decades, neuroscientists have used every suitable new technology that came their way to fine-tune the definition of what constitutes a distinct cell type in these classes. Cells that superficially look the same, researchers realized, could be different cell types, depending on their connections with other brain cells or regions, or their electrical properties.
At the same time, researchers were collecting data on how neurons connect together in networks and what the networks’ properties are. (When the HBP launched, it focused on generating the algorithms and computing power to help researchers to simulate how these networks might function together.)
From the 1990s, researchers began to study genes’ activity in different cell types and how their expression reflected their properties.
In 2006, the Allen Institute created a gene-expression atlas showing where in the mouse brain each of its roughly 21,000 genes are expressed. It took 3 years for around 50 staff to build the Allen Brain Atlas one gene at a time — and its value was instantly recognized by the neuroscience community. It is updated regularly and continues to be widely used as a reference, helping scientists to locate where their gene of interest is expressed or to study the role of a particular gene in a disease.
Still, the community wanted more. “We wanted to be able to see every gene that is expressed in every cell at the same time,” says Hongkui Zeng, director of the Allen Institute for Brain Science. The different patterns of gene expression in individual cells would allow researchers to define which type of cell they were — an ambitious task because the mouse brain contains more than 100 million cells, two-thirds of which are neurons. (The human brain is three orders of magnitude larger, with more than 170 billion cells, of which half are neurons.)
A game-changing technology that emerged in the mid-2000s promised to help achieve this. Scientists had developed a way of sequencing RNA in single cells, a technique that has transformed all areas of biology in the past decade. A cell’s transcriptome — the RNA that represents a read-out of all its protein-coding genes — is an indicator of which proteins the cell is making at a given time.
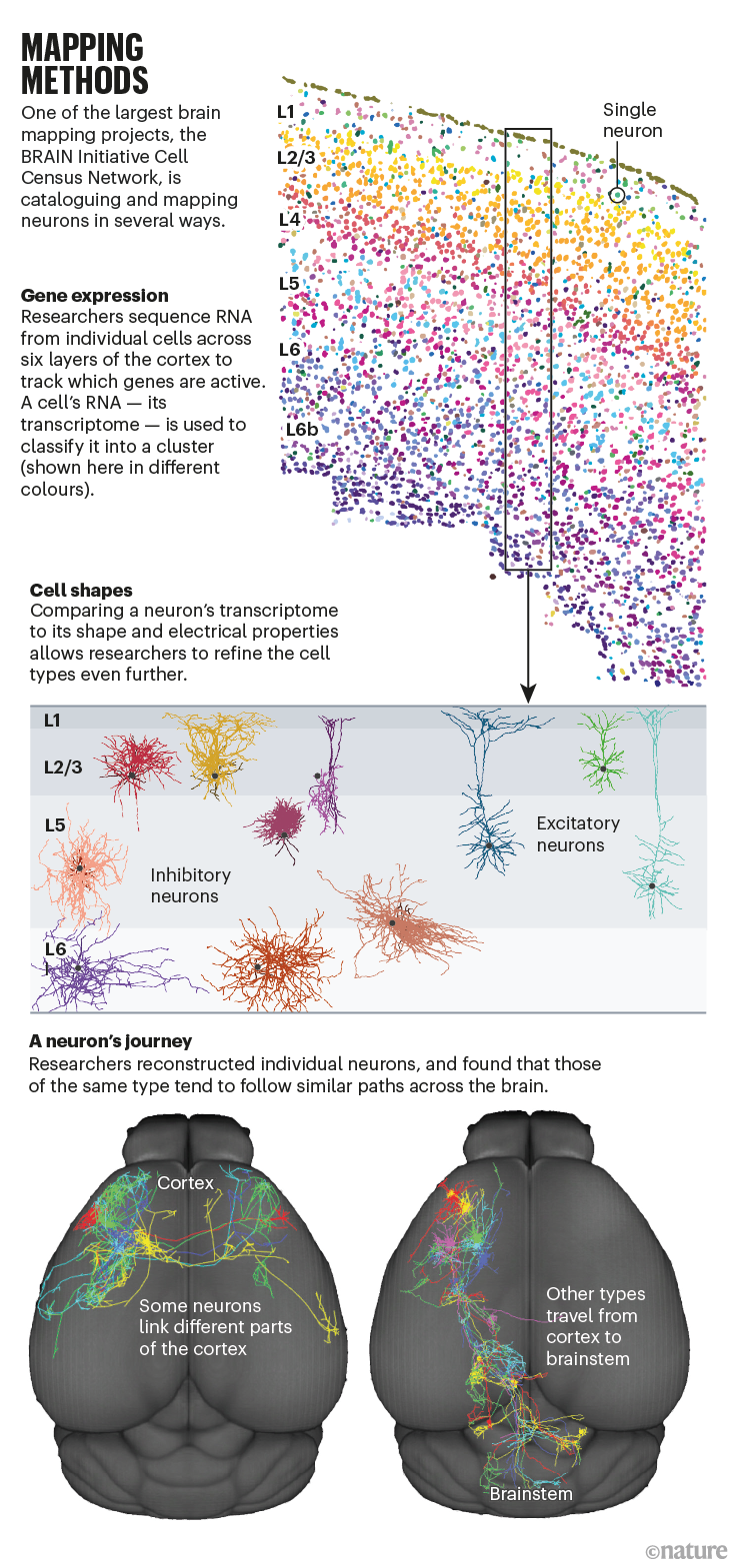
In 2017, the BRAIN Initiative decided to finance a network of laboratories, including the Allen Institute as an important player, to use this method and other, even newer, technologies to map and characterize the cell types in the whole brain (see ‘Mapping methods’). Two years later, the BICCN scientists were ready to begin their effort.
Sequencing frenzy
For their pilot project, the researchers chose a modest target: a small corner of the mouse brain known as the motor cortex, which processes information about the planning and execution of movement. The motor cortex has unambiguous counterparts in all mammals, making it possible to compare results from mice, humans and other species. They measured the RNA content in more than 1.1 million individual cells and analysed how it clustered5. The effort took around ten BICCN scientists just three months.
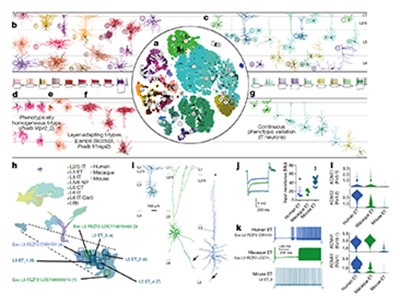
They found 56 distinct clusters, each considered to represent a different cell type. One big question is whether a cell’s genetic classification matches up with everything else it does, including how it fires, what shape it has and where it projects, says the Allen Institute’s Ed Lein.
So far, it does seem to match, he says. Lein led a parallel BICCN project that analysed fresh brain tissue removed from an individual during surgery for brain cancer, using a particularly powerful method called patch–seq that allows three distinct types of measurement from a single cell. The technique uses a special glass pipette that clamps to the cell’s membrane, records its electrical activity, infuses a dye into the cell so that its anatomy can be visualized and then sucks out the cell’s contents for transcriptome analysis.
The team showed that cells with a common transcriptomic pattern also shared the same distinct shape and firing patterns6. “This indicates that transcriptomics can serve as a Rosetta stone for interpreting cell diversity and predicting cellular properties,” says Lein.
Scientists outside the collaboration have already taken inspiration from the results, particularly the discovery that neurons of a single class can be so different from each other.
Two years ago, neuroscientist Anne Churchland at the University of California, Los Angeles, started to design a set of experiments in mice to see whether that diversity mattered in excitatory neurons. Her early results, which have not been peer reviewed7, suggest that it might: different excitatory neurons fire at different times as mice perform a listening task. “We are at a really exciting stage,” she says.
Bigger brains
In the next phase of the cell census, the teams will focus more on larger brains. Some of this work has already begun. RNA sequencing of post-mortem marmoset and human brains has revealed remarkable consistency in cell types across species6. What, then, accounts for the markedly superior cognitive power of humans?
“The major take-home message from these studies is that the general blueprint of cell types is conserved across species,” says Lein. “Still, you can find evidence for species specializations that are quite significant, even if they are just variants of a theme.” The BICCN transcriptomic studies show a greater diversity of cell types in the human brain than in the mouse brain, particularly in neurons that are most recently evolved. One of these corresponds to a type of neuron known to be selectively depleted in Alzheimer’s disease8.
Furthermore, the ratio of different cell types varies between humans, marmosets and mice. These properties might help us to better understand human-specific diseases, says Lein.
Lein is now performing transcriptomic analysis on 100 post-mortem brains from people who had Alzheimer’s disease when they died. Comparing these disease-specific maps with the reference maps from the BICCN will more systematically reveal the most vulnerable of our cells, he says.
Another difference highlighted by the BICCN studies is the large shift in the balance of excitatory and inhibitory neurons in the cortex between mice, marmosets and humans. The ratio is 2:1 in humans, compared with 3:1 in marmosets and 5:1 in mice6. That’s a surprising and rather mysterious finding, notes Lein. “These cumulative differences could lead to profound changes in how the human cortex is organized and functions,” he says.
What makes the human brain special will come down to differences in the cellular diversity, the proportions of the cell types, the wiring of the brain and probably much more, says neuroscientist John Ngai at the University of California, Berkeley, who heads the US BRAIN Initiative. “There’s no simple answer to this age-old question.”
From maps to medicine
One of the next steps for the BRAIN Initiative, says Ngai, will be to build tools that selectively target particular cell types in circuits relevant to disease and deliver therapeutic molecules that can tune those circuits up or down.
The targeting method that researchers are particularly excited about relies on the BICCN’s discovery of short snippets of DNA that are unique to individual cell types9. These short sequences can serve as markers for those cell types, allowing researchers to create mouse strains in which they can target different cells and manipulate the cells’ activity10 — and therefore the activity of the associated circuits. Both basic science and medicine stand to benefit. “The ability to target every cell in the brain will be a great support for fundamental research,” says Edvard Moser at the Kavli Institute for Systems Neuroscience in Trondheim, Norway, who shared the 2014 Nobel Prize in Physiology or Medicine for his work on navigation in the brain.
These tools will also be “enormously important” for gene therapy, a treatment that replaces a gene that is missing or broken, says Botond Roska at the Institute for Molecular and Clinical Ophthalmology in Basel, Switzerland. Roska is testing the world’s first optogenetic therapy — in which light-sensitive proteins are inserted into neurons in the retina — in people with a certain type of blindness. He says it took him 19 years from deciding to identify the appropriate cells in the retina to publishing the successful treatment of the first individual11 in May. The BICCN activities will speed up research for scientists working on other brain areas in the future, he says.
Developers of drugs for psychiatric and neurological conditions need to consider the cell type, but until now this has not been possible, says Gordon. “Right now, we are throwing drugs at all of the cells at once without knowing which cells they affect — that’s why so many of our treatments in psychiatry and neurology have significant side effects.”
Zooming out
Knowing the brain’s parts is one thing. Knowing how they work together is another. Some of the large brain projects, along with several independent research groups around the world, are working out the spatial organization of cell types and their connections — known as connectomes — for many species, including mice and humans.
To do this, scientists stain the brain and then slice it into ultrathin layers, images of which are captured by an electron microscope. Then they stack the images together and use artificial intelligence to trace the 3D path of each cell. The resolution is so fine that it exposes every synapse — tiny structures in a cell’s membrane that forge chemical connections with other cells.
Scientists at Janelia Research Campus in Ashburn, Virginia, expect to complete the fruit-fly connectome next year. The scale of the endeavour required for larger species means that further full connectomes are years, if not decades, away. The BICCN plans to create a 3D anatomical map of the entire mouse brain using high-resolution electron microscopy — providing the billion-fold magnification needed to see the cells’ inner workings. Scientists working on the Japan Brain/MINDS Project are tracing the marmoset connectome, and a handful of groups outside the government-backed big-brain projects, including three in different institutes of Germany’s Max Planck Society, are working on connectomes of other large mammals.
Current efforts are limited by the computational power required to reconstruct even the smallest specks of brain tissue. But these small volumes of connectome are still useful, says Moritz Helmstaedter, a director of the Max Planck Institute for Brain Research in Frankfurt, Germany, because “we can start to ask exciting questions about how our circuits are shaped by our individual experience or evolutionary predisposition”.
Brain barriers
Most neuroscientists think that big mapping projects are key to the field’s future, but some remain cautious. Neurophysiologist Tony Movshon at New York University is sceptical that detailed knowledge of cell types and connectomes will be of immediate help. “We already knew some cell types from morphology and other classifications before anyone did a transcriptomic analysis, and we are still completely at sea,” he says. “Knowing that there are more genetically distinct types is not going to be very helpful in the near term for understanding how a circuit works.”
But tools that enable the tagging or manipulation of particular cell types will be “terrific”, he says. “We would have learnt so much more if we had known more about the cells we are recording from.”
Movshon had also been a sceptic of the Human Genome Project (HGP) when it was launched in 1990, but, again, he says, the spin-offs from the project — including the tools that enabled the cell census work — were transformative.
Scientists see many other parallels between the BICCN and the HGP efforts, in terms of scientific insights as well as research tools. Once the draft of the human genome was completed in 2001, researchers realized that humans do not have significantly more genes than mice do. They discovered that, to make sense of how the system worked, they needed more than just the basic catalogue of parts. They needed extra layers of information about how and when the genes are expressed, and how genes influence each other and interact with the environment.
The challenge is similar for the BICCN, but its scope will ultimately dwarf that of the HGP, says Huang. “The genome is just one type of information, a string of nucleotides; the cell type atlas is many different types of information.”
As the stream of data from the cell census continues, researchers are working on ways to combine the information into a ‘common coordinate framework’ — a sort of reference brain for a particular species. In this way, multiple types of information can be pulled out from a single location.
The HBP’s EBRAINS platform is creating its own common coordinate framework. It’s a huge but essential computational challenge to link different types of biological information together in the same space, so that studies in — and eventually between — species can be compared, says Wim Vanduffel, a neurophysiologist at the Catholic University of Leuven in Belgium, who is part of the HBP effort. “Common frameworks serve as anchoring points,” he says.
The HBP and the BICCN are discussing how to link their data together. “The BICCN is bottom-up and we are top-down,” says Katrin Amunts, a neuroscientist at the Heinrich Heine University of Düsseldorf, Germany, and the HBP’s scientific research director.
The ultimate goal is to build an observatory that can integrate data from all these projects into one grand, unified picture. Four years ago, with that in mind, researchers at the big-brain projects got together to create the International Brain Initiative, a loose organization with the principal task of helping neuroscientists to find ways to pool and analyse their data.
On the distant horizon lies the prospect of hacking the brain’s circuits to remedy brain disorders, says Koch.
“The brain is the most staggeringly complex piece of highly active matter in the Universe,” he says. “There is no magic bullet to cracking how it works, but having the basic hardware will lead to a mechanistic understanding of its circuits.”
Article From & Read More ( How the world's biggest brain maps could transform neuroscience - Nature.com )https://ift.tt/3Ajjsgq
Health
Bagikan Berita Ini















0 Response to "How the world's biggest brain maps could transform neuroscience - Nature.com"
Post a Comment